
Synthesis mechanism and characteristics of nanocapsules
Synthesis of HNS
The synthesis of HNSs utilized TEOS, a compound chosen for its well-established hydrolysis and condensation properties, both of which are crucial for forming a stable silica network. The process begins in an alkaline medium, facilitated by ammonia, which initiates the hydrolysis of TEOS. During hydrolysis, the OR groups in TEOS are replaced by OH groups, producing Si(OH)₄ and alcohol. This preparation step is vital for the subsequent condensation phase. In the condensation reaction, Si(OH)₄ molecules interact, forming Si–O–Si bonds, with water as a byproduct. The reactions involved are as follows:
-
1.
Hydrolysis of alkoxysilanes
Si(OR)4 + 4H2O → Si(OH)4 + 4ROH.
-
2.
Condensation reaction
(OR)3Si–OH + HO–Si(OR)3 → (OR)3Si–O–Si(OR)3 + H2O.
The cationic surfactant, CTAB, plays an essential role in the process by forming micelles in the aqueous medium. These micelles, characterized by their positively charged surfaces, attract the negatively charged hydrolyzed and condensed silica species, leading to the deposition of a silica shell around the micelles. This interaction is critical for controlling the size and shape of the nanocapsules. The positively charged CTAB micelles serve as a template for silica deposition, ensuring the nanocapsules maintain a uniform spherical structure, which is important for applications requiring consistency in particle size and morphology.
The success of this synthesis method is evident in the uniformity of the produced nanocapsules. Precise control over the hydrolysis and condensation reactions, combined with CTAB’s templating role, ensures that the HNSs exhibit structural integrity. This stability is especially valuable for controlled-release systems and other applications where consistent performance is critical.
Mechanism of UF–HPN synthesis
The synthesis of UF–HPNs follows a polymerization process where urea reacts with formaldehyde, resulting in the formation of hydroxymethyl urea derivatives. These derivatives undergo further polymerization and cross-linking to form a stable polymer network. The process consists of three key stages: (i) formation of hydroxymethyl derivatives: urea reacts with formaldehyde to produce hydroxymethyl urea derivatives, which act as intermediates during the polymerization process. (ii) Polymerization: the hydroxymethyl derivatives further react, forming long polymer chains. This stage constructs the fundamental polymer framework that will encapsulate the EOs. (iii) Cross-Linking: the polymer chains undergo cross-linking, creating a three-dimensional network. This cross-linking step is crucial for providing mechanical stability to the nanocapsules, allowing them to withstand environmental variations without degrading.
Scheme 1illustrates the reaction pathway for the UF polymer synthesis, showing the formation of hydroxymethyl derivatives, the subsequent polymerization, and the critical cross-linking phase that imparts stability and functionality to the nanocapsules34.
The synthesis and cross-linking of urea-formaldehyde polymers under both alkaline and acidic conditions34.
UF was selected as the encapsulating material due to its ability to form a rigid and durable polymer shell, particularly suited for protecting volatile compounds such as EOs. The cross-linked structure not only ensures mechanical durability but also acts as a barrier, regulating the release of the encapsulated material. This makes the nanocapsules ideal for applications requiring a controlled and extended-release profile.
Adsorbent characterizations
FT–IR spectroscopy provides critical insights into the chemical structure and qualitative encapsulation efficiency by identifying key functional groups within the nanocapsule matrices. The spectral data confirmed the successful encapsulation of Sage and Thyme EOs in both HNS and UF–HPNs, retaining the chemical integrity of the oils and nanomaterials. The FT–IR spectrum of HNS (Fig. 1a) exhibited characteristic absorption peaks between 1000 and 1130 cm−1, corresponding to the asymmetric stretching vibrations of Si–O–Si bonds, a critical structural component of the silica network. The peak at 963 cm−1 is attributed to Si–OH stretching, confirming the presence of surface silanol groups, while the broad band around 3228 cm−1, associated with O–H stretching vibrations, indicates hydroxyl groups. These findings are consistent with previous studies confirming the structural integrity of nanosilica frameworks for adsorptive applications. The FT–IR spectra of nanosilica encapsulating Thyme and Sage oils (Fig. 1b and c) displayed similar peaks for Si–O–Si bonds, confirming that the encapsulation process did not disrupt the silica network. Additionally, new peaks were observed in the 2961–2925 cm−1 range, corresponding to C–H stretching vibrations, which are indicative of the presence of aliphatic hydrocarbons from the EOs. These peaks, rather than those corresponding to C = C bonds, suggest that the encapsulation successfully retained the organic compounds from the EOs. This observation confirms that the encapsulation was successful and that the stability of both the EOs and the silica matrix was maintained throughout the process.
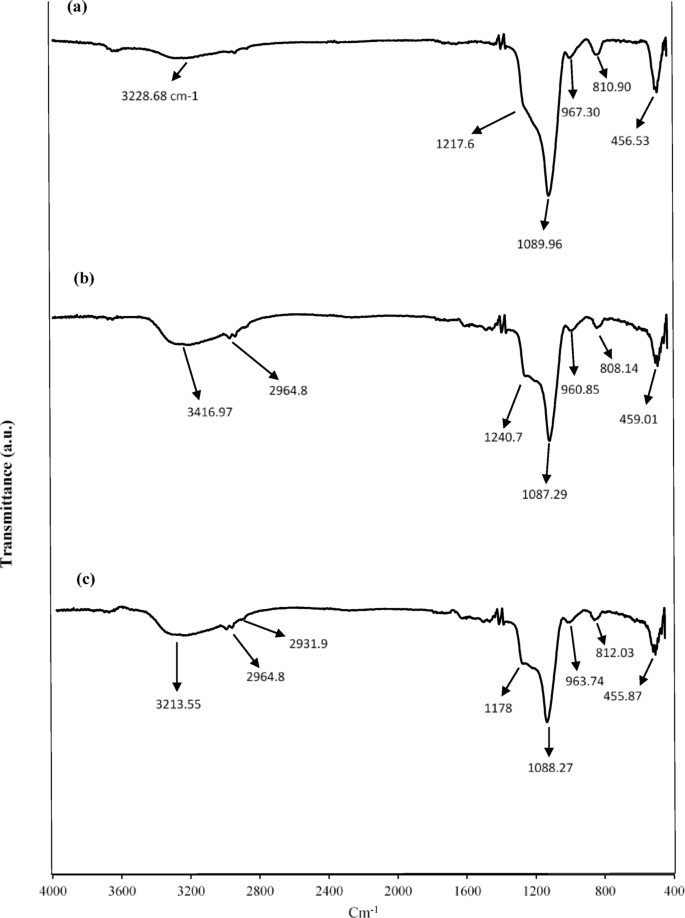
FT–IR of (a) HNS; (b) HNS–Thyme–EO; (c) HNS–Sage–EO.
The FTfIR spectra for UF–HPNs (Fig. 2a) revealed characteristic bands around 1636 cm⁻¹ for C = O stretching and 1563 cm⁻¹ for N–H bending, typical of secondary amide bonds, confirming the successful formation of the polymer matrix. In the presence of Sage and Thyme oils, peaks in the 2960–2925 cm⁻¹ range showed slight shifts, indicating interactions between the polymer matrix and the EOs (Fig. 2b and c). These shifts suggest hydrogen bonding between the oils and the polymer, as supported by previous studies highlighting such interactions in polymer-based encapsulation systems. The FT–IR results confirmed theation of EOs within both silica and HPNs, with no significant disruption of the nanocapsule matrix. This preservation of chemical structure is crucial for maintaining the functionality and controlled release properties of the encapsulated oils. The FTIR spectra of pure essential oils are also given in the Supplementary material in Figure S1.
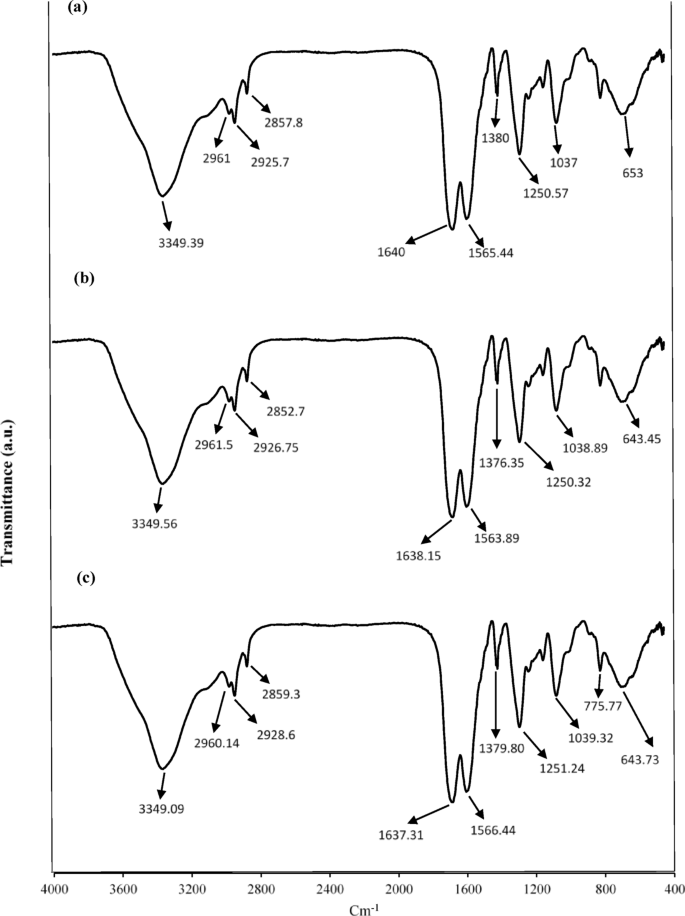
FT–IR of (a) HPNs (b) HPNs–Thyme–EO (c) HPNs–Sage–EO.
SEM images revealed that HNSs exhibit a spherical morphology with smooth surfaces, as shown in Fig. 3a. The uniform size distribution, ranging from 55.0 nm to 84.2 nm for Sage oil encapsulation (Fig. 3b and d) and 43.2 nm to 66.7 nm for Thyme oil encapsulation (Fig. 3c and e), confirms successful formation and encapsulation. These findings align with previous studies that highlight the ability of HNSs to provide high surface area and uniform morphology, which are crucial for enhancing encapsulation efficiency and retention of volatile compounds such as essential oils (EOs). Similar morphological characteristics have been reported in silica-based nanocarriers, where their well-defined spherical structure contributes to improved stability and controlled release properties35.
The SEM image of UF–HPNs (Fig. 3f) also displayed a spherical morphology, consistent with previous observations of polymer-based nanocapsules used for controlled release applications. The particle size of UF–HPNs encapsulating Thyme oil (Fig. 3g and i) ranged from 140.5 nm to 175.2 nm, while those encapsulating Sage oil (Fig. 3h and j) ranged from 118.7 nm to 267.0 nm. The smooth, crack-free surfaces indicated effective encapsulation without aggregation, a feature commonly observed in polymeric nanocapsules designed for sustained release of bioactive compounds. Studies have demonstrated that polymer-based nanocarriers typically exhibit a more compact and uniform structure compared to inorganic matrices, contributing to their enhanced stability and controlled diffusion profiles36.
The morphological consistency and smooth surfaces observed in both nanocapsule systems further confirm the efficiency of the encapsulation process, qualitatively. The absence of surface deformations or structural disruptions suggests that these nanocapsules are well-suited for applications where stability, protection, and prolonged release of encapsulated materials are critical. These observations are in agreement with prior research on nanocapsule systems used for drug delivery and agricultural formulations, where particle uniformity and integrity play key roles in ensuring optimal performance37.
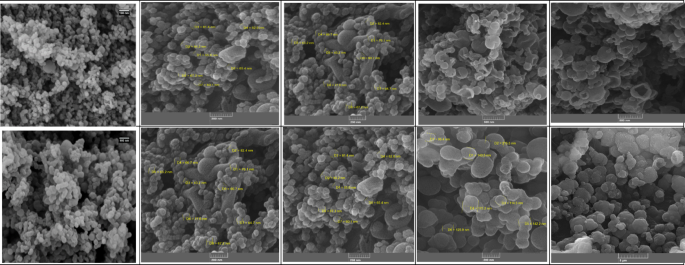
SEM images of (a) HNS, (b) HNS–Sage–EO (immersed) (c) HNS–Thyme–EO (immersed), (d) HNS–Sage–EO (in-situ), (e) HNS–Thyme–EO (in-situ), (f) HPNs, (g) HPNs–Thyme–EO (immersed), (h) HPNs–Sage–EO (immersed), (i) HPNs–Thyme–EO (in-situ), (j) HPNs–Sage–EO (in-situ).
Dynamic Light Scattering (DLS) measurements were conducted to evaluate the hydrodynamic size (Z-average) and polydispersity index (PDI) of the encapsulated essential oil samples in different formulations and preparation methods. The results, summarized in Table 1, provide insights into the size distribution and uniformity of the nanocapsules, which are critical parameters for their performance in various applications.
The hydrodynamic size (Z-average) of the nanocapsules varied significantly based on the type of essential oil (Thyme or Sage), encapsulation method (immersed or in-situ), and capsule matrix (HNS or HPN). Thyme-immersed HNS nanocapsules exhibited the smallest hydrodynamic size (233.80 ± 11.7 nm) with a moderate PDI of 0.26, indicating a relatively narrow size distribution. Sage-immersed HNS nanocapsules showed a larger Z-average of 320.48 ± 16.0 nm and a higher PDI (0.30), suggesting greater heterogeneity in size.
In the in-situ encapsulation method, the Z-average for both Thyme and Sage formulations increased significantly, with Thyme reaching 423.67 ± 21.2 nm and Sage measuring 324.85 ± 16.2 nm. The PDI values remained moderate (0.26 and 0.19, respectively), indicating a controlled particle size distribution despite the increase in size.
On the other hand, Thyme and Sage-immersed HPN nanocapsules demonstrated much larger hydrodynamic sizes (608.22 ± 30.4 nm and 672.92 ± 33.6 nm, respectively) compared to their HNS counterparts. These samples also exhibited extremely low PDI values (0.05 and 0.02), signifying highly uniform particle size distributions.
The in-situ method further increased the hydrodynamic size for both Thyme and Sage HPN samples, with Thyme reaching 688.00 ± 34.4 nm and Sage extending to 794.23 ± 39.7 nm. While the PDI values remained low for Sage (0.07), the Thyme sample showed a slight increase to 0.19, suggesting a broader size distribution in the in-situ formulation.
The differences in Z-average values can be attributed to several factors: (i) EO Type: The molecular composition and polarity of Thyme and Sage EOs influence their interactions with the nanocapsule matrix, potentially affecting swelling and aggregation behavior; (ii) Encapsulation method: In-situ encapsulation may lead to better dispersion and uniformity, whereas post-synthesis immersion could result in partial aggregation or surface adsorption, thereby altering the hydrodynamic size; (iii) Capsule matrix: The structural differences between HNS and HPNs, including porosity, surface charge, and rigidity, impact their interaction with the surrounding medium, leading to variations in hydration layers and size distribution.
This analysis underscores the importance of tailoring encapsulation parameters to optimize the performance of essential oil-loaded nanocapsules for specific applications.
Release test results
The release profiles of EOs encapsulated within nanocapsules were monitored over a 102–day period to evaluate their potential for controlled release applications (Fig. 4; Table 2). Controlled release is a key factor in determining the suitability of these nanocapsules for various industrial, agricultural, and biomedical uses, as it directly affects the delivery efficiency and duration of the bioactive compounds.
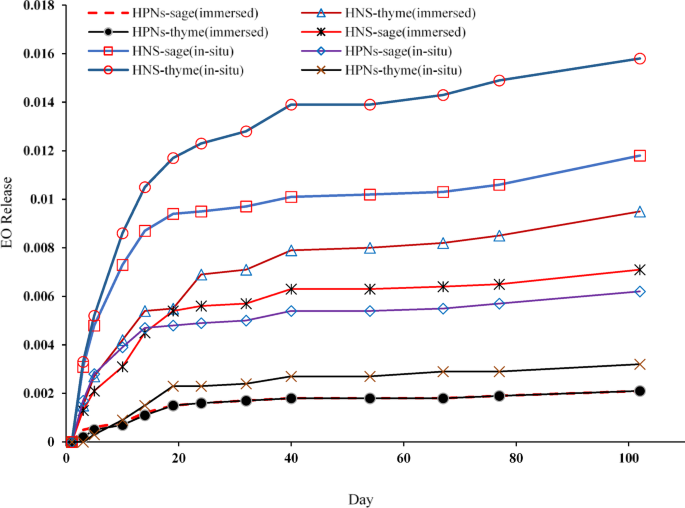
Release profile of essential oils from silica and polymer nanocapsules over 102 days, comparing immersed and in-situ methods for Thyme and Sage oils.
HNSs demonstrated faster and higher release rates than polymer-based nanocapsules. This difference can be attributed to the porous structure of the silica matrix, which enables rapid diffusion of the encapsulated EOs. Among the different HNSs, those produced via in-situ methods exhibited the most rapid release of EOs due to their larger size and increased loading capacity. The porous structure of the silica provides numerous diffusion pathways for EOs, enhancing their release rate.
Several studies support these findings, highlighting that the large surface area and porosity of HNSs significantly enhance their release efficiency. This is particularly beneficial for applications requiring rapid diffusion of active compounds, such as in agricultural biopesticides, where a quick response to pest outbreaks is essential. In such cases, the fast release of EOs from HNSs ensures a swift and effective action against pests.
Conversely, HPNs exhibited a slower, more controlled release profile compared to their silica counterparts. This slower release is due to the denser, less porous nature of the polymer matrix, which hinders the diffusion of the encapsulated EOs. The more uniform and stable encapsulation environment within the polymer matrix forms an effective barrier to diffusion, thus providing sustained release over an extended period.
This observation aligns with other studies that demonstrate the effectiveness of HPNs in providing a prolonged and steady release, making them ideal for applications such as drug delivery systems and long-term agricultural treatments. The lower polydispersity index (PDI) of HPNs indicates their higher stability, which enhances their ability to deliver a controlled and consistent release over time.
Across all types of nanocapsules, Thyme EO exhibited a higher release rate compared to Sage EO. This difference can be explained by the intrinsic properties of the oils, such as molecular weight, volatility, and interaction with the encapsulating matrix. Thyme EO, with its lower molecular weight and higher volatility, diffuses more readily through both silica and polymer matrices, resulting in a faster release profile.
On the other hand, Sage EO, characterized by a higher molecular weight and lower volatility, has a stronger interaction with the encapsulating matrices, leading to a slower release.
Sage essential oil (EO), which contains a higher proportion of sesquiterpenes and diterpenes compared to ThymeEO, exhibits lower volatility due to the increased molecular weight of these components. Studies have shown that essential oils with larger molecular structures tend to have lower vapor pressures, reducing their tendency to evaporate rapidly38. This characteristic leads to stronger interactions with encapsulating matrices, as larger and less volatile molecules exhibit greater affinity for polymeric and inorganic hosts through hydrogen bonding and van der Waals interactions39. Consequently, encapsulated SageEO demonstrated a slower release rate, aligning with previous reports indicating that oils with higher molecular weights diffuse more slowly through nanoscale carriers due to steric hindrance and matrix retention effects40. The controlled release of Sage EO observed in this study is consistent with these established principles, reinforcing the importance of molecular weight and volatility in determining release kinetics.
Similar results have been reported in other research, where differences in EO composition significantly influence their release kinetics when encapsulated in nanomaterials. Adjusting the release pattern of EOs and the encapsulation methods allows for optimizing the delivery system to suit the specific requirements of the intended application.
Equilibrium adsorption analysis of EOs
Using Eq. 1, the equilibrium adsorption capacity (qe) was calculated from the experimental data for Thyme and Sage EOs adsorbed onto the synthesized hollow nanocapsules.
$${q}_{e}=\frac{{C}_{0}-{C}_{e}}{m}V$$
(1)
where qe (mg/g) is the amount of EO adsorbed on the HNS or HPN at considered equilibrium concentration, C0 (mg/L) is the initial concentration of the EO in solution, Ce (mg/L) is the equilibrium concentration of the EO in solution, V (L) is the volume of the EO solution and m (g) is the mass of adsorbent used.
The equilibrium data were analyzed in accordance with the Langmuir, Frundlich and Temkin sorption isotherm models. A nonlinear regression analysis was applied to evaluate the adsorption parameters for all isotherms. All of the models are listed in Table 3.
The adsorption equilibrium behavior of both EOs on HNS and HPNs was assessed to evaluate their effectiveness in encapsulating EOs for controlled release applications.
The equilibrium adsorption data for Thyme EO on HNSs at 25 °C are detailed in Table S1 (in Supplementary material), while Fig. 5a shows the relationship between the equilibrium concentration (Ce) and the adsorbed amount (qe). The adsorption capacity (qe) of Thyme EO on HNSs increased with the initial concentration of the EO, reaching a maximum of 5.32 mg/g at a Ce of 128.06 ppm. The adsorption isotherm showed Langmuir-like behavior, implying monolayer adsorption on a homogeneous surface. This high adsorption capacity is attributed to the porous structure and large surface area of the HNSs, which provide numerous active sites for adsorption.
Similarly, the equilibrium adsorption of Thyme EO on HPNs at 25 °C (Fig. 5b) followed the same increasing trend, although the maximum adsorption capacity reached only 3.63 mg/g at a Ce of 98.18 ppm. This lower capacity compared to HNSs is likely due to the denser structure of the polymer matrix, which limits the number of active sites for adsorption. Despite this, the HPNs exhibited sufficient adsorption capacity for applications requiring controlled release.
HNSs showed a higher adsorption capacity than HPNs at lower concentrations. At an initial concentration of 24.00 ppm, HNSs achieved a qe of 0.61 mg/g, while HPNs only reached 0.19 mg/g. The porous structure of silica facilitates more efficient uptake of Thyme EO, especially at low concentrations, making it better suited for applications needing rapid adsorption. As the concentration of Thyme EO increased, HNSs continued to outperform HPNs. At a Ce of 94.89 ppm, HNSs achieved an adsorption capacity of 4.18 mg/g, whereas HPNs reached 3.63 mg/g. This difference in performance is attributed to the porous nature of the silica, which offers faster and more efficient adsorption.
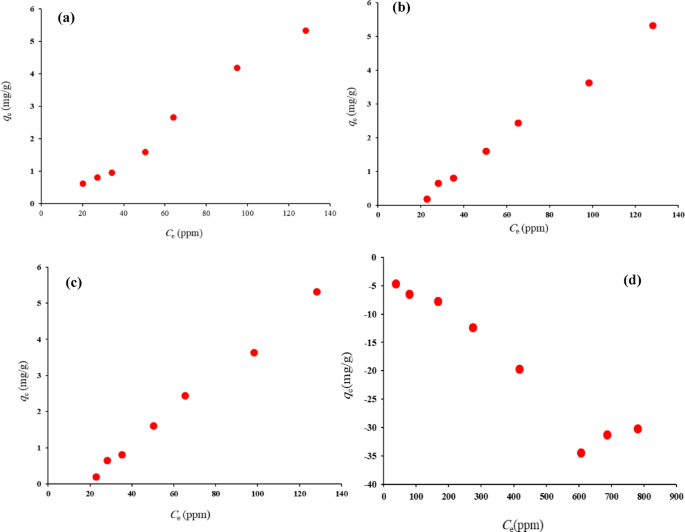
Equilibrium adsorption isotherm of (a) Thyme–EO on HNS; (b) Thyme–EO on HPNs; (c) Sage–EO on HNS; (d) Sage–EO HPNs, at 25 °C.
A similar trend was observed for the adsorption of Sage essential oil onto HNS (Fig. 5c), while the adsorption behavior of Sage EO on HPNs (Fig. 5d) displayed significant data scatter, preventing clear trends. This inconsistency may stem from weaker interactions between Sage EO and the nanocapsule surfaces, potentially due to the molecular structure of Sage oil or other factors not explored in this study.
At higher concentrations, both HNS and HPNs converged to the same maximum adsorption capacity of 5.32 mg/g at a Ce of 128.06 ppm. This suggests that, at elevated concentrations, both types of nanocapsules saturate their adsorption sites and reach similar performance levels. Despite silica’s superior performance at lower concentrations, both materials demonstrated comparable adsorption capacities at maximum loading. The porous structure of HNSs provides a larger surface area and more active sites for adsorption, contributing to their higher adsorption capacity, especially at lower concentrations. This makes HNSs ideal for applications requiring rapid and high-capacity adsorption. The denser structure of HPNs slows the adsorption process by limiting diffusion, but this also makes them better suited for applications needing a sustained and controlled release over time.
The adsorption of Thyme EO onto both HNS and HPNs was further evaluated using three isotherm models: Langmuir, Freundlich, and Temkin. The parameters for each model are presented in Table S2 (in Supplementary material), with the corresponding adsorption isotherms shown in Fig. 6a and b. The Langmuir isotherm assumes monolayer adsorption on a finite number of homogeneous adsorption sites. The qm values for HNS and HPN were − 20.01 mg/g and − 8.88 mg/g, respectively, indicating a higher adsorption capacity for HNS. This aligns with the porous structure of silica, which offers more active sites. However, the negative KL values suggest the need for further investigation into the adsorbate-adsorbent interactions.
The KF parameter, which reflects the adsorption capacity, was determined to be 0.016 for HNS-based nanocapsules and 0.0075 for HPN-based nanocapsules. This result suggests that HNS-based nanocapsules have a higher adsorption capacity compared to HPN-based nanocapsules, indicating that the HNS matrix provides more favorable surface properties for adsorption.
The AT values of the Temkin model for HNS and HPNs were 0.048 and 0.0412, respectively, indicating weak adsorbent-adsorbate interactions for both materials. The slightly higher AT value for HNS implies stronger interactions than in HPNs, corresponding with HNS’s higher adsorption capacity.
The values of R2 and RMSE as two common error functions are listed in Table S3 (in Supplementary material). The values of RMSE about Freundlich model for Thyme were smallest and the value of R2 was greatest, among the three isotherms.
In summary, HNSs exhibited superior adsorption performance due to their larger surface area and porous structure, making them ideal for rapid and high-capacity adsorption. HPNs, while displaying lower initial adsorption, showed more controlled and sustained release, advantageous for applications requiring prolonged efficacy. These findings highlight the need to select appropriate materials based on the desired application, whether it be for rapid adsorption or controlled release.
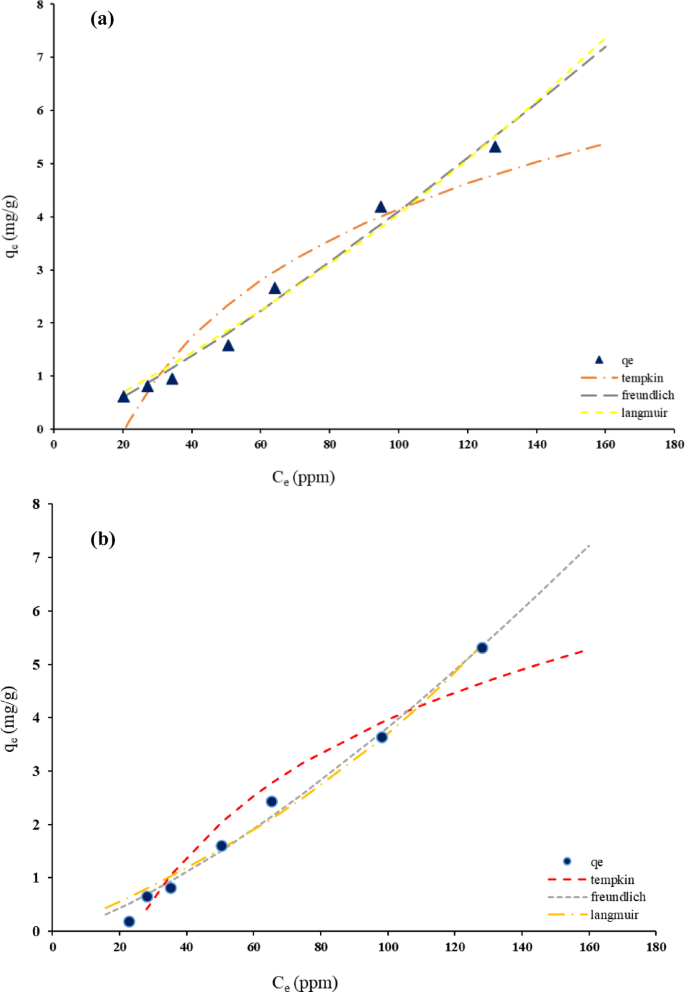
Thyme–EO adsorption isotherms on (a) HNS adsorbent, (b) HPNs adsorbent.
Kinetic studies
The adsorption kinetics of Thyme and Sage EOs on synthesized hollow nanocapsules were studied by calculating the amount of oil adsorbed over time (qt) using Eq. 2:
$${q}_{t}=\frac{{C}_{0}-{C}_{t}}{m}V$$
(2)
Experimental data are displayed in Table S4 (in Supplementary material) and Fig. 7a and d, revealing the time-dependent adsorption of EOs on both HNS and HPN. The adsorption kinetics of Thyme EO on HNSs, depicted in Fig. 7a, show a steady increase in adsorption rate over time until equilibrium is reached. The kinetic data for Thyme EO on HPNs (Fig. 7b) suggest a slightly different adsorption pattern, likely due to variations in the polymer structure affecting the surface properties and adsorption behavior. For Sage EO, the adsorption kinetics on HNSs are presented in Fig. 7c. This shows a slower adsorption process compared to Thyme oil, which may be attributed to differences in the molecular properties of Sage oil and its interaction with the silica surface. Sage EO adsorption on HPNs (Fig. 7d) also shows slower kinetics, indicating sustained adsorption over time. These findings highlight that both the type of nanocapsule material and the EO used significantly influence the adsorption rate. HNSs tend to have a faster adsorption process for Thyme EO, while HPNs offer a more controlled release. Sage EO generally adsorbs more slowly than Thyme oil across both nanocapsule types, suggesting that different formulations could be tailored for specific applications requiring different release rates.
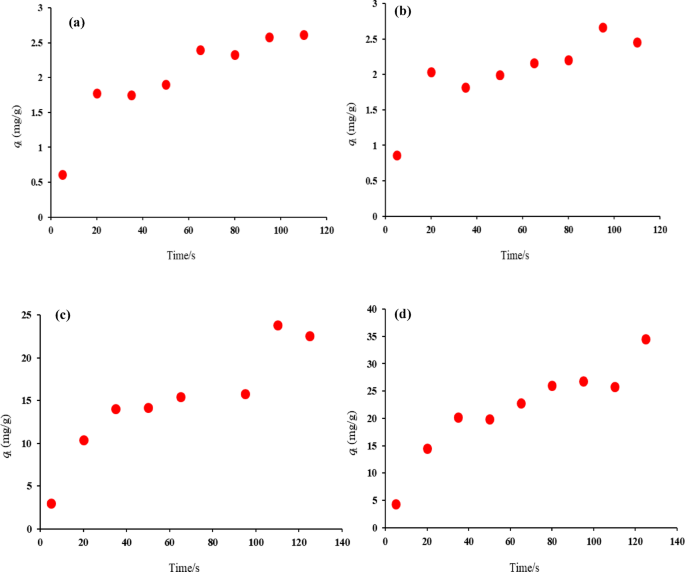
Kinetic adsorption of (a) Thyme–EO on HNS, (b) Thyme–EO on HPNs, (c) Sage–EO on HNS, (d) Sage–EO on HPNs at 25 °C.
The adsorption kinetics of EOs on HNS and HPNs were further analyzed using various kinetic models (PFO, PSO, Elovich, and intra-particle diffusion) via nonlinear regression methods. All of the models are listed in Table S5 (in Supplementary material) and the values of parameters obtained by different kinetic models are summarized in Tables S6 to S9 (in Supplementary material), reveal that the pseudo-second-order model provides the best fit for Thyme oil on HNSs (Fig. 8a), while the Elovich model offers a better fit for HPNs (Fig. 8b).
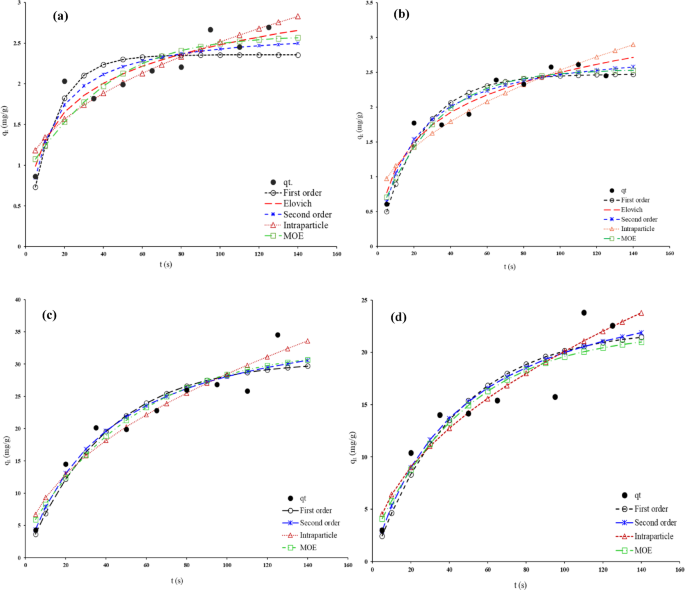
Typical fitting data with various kinetic models for adsorption of (a) Thyme–EO on HNS, (b) Thyme–EO on HPNs, (c) Sage–EO on HNS, (d) Sage–EO on HPNs at 25 °C.
For Sage EO adsorption on HNSs (Fig. 8c), the pseudo-second-order model again provides the best fit, while the intra-particle diffusion model gives a more accurate representation for adsorption on HPNs (Fig. 8d). These variations in model fitting underscore the complexity of the adsorption process, which may involve multiple mechanisms.
The values of different errors are listed in Table 4. For Thyme EO adsorption kinetics on HNS, the values of error functions about pseudo-second order kinetic model were smallest among the five kinetics models. The obtained data show that the pseudo-second order kinetic model explains the adsorption in a better way. On the other hand, examination of the kinetic error functions of the adsorption of Thyme on HPN shows that its adsorption follows the Elovich equation. For Sage EO adsorption kinetics, the intra-particle diffusion model shows better error functions for both HNS and HPN adsorbents.
Enzyme peroxidase inactivation
The impact of encapsulated EOs on peroxidase enzyme activity was studied to explore their potential applications in biological systems. The enzyme peroxidase plays a crucial role in plant defense mechanisms, food processing, and preservation. Figure 9a and b illustrate the effects of Sage and Thyme EOs on the activity of peroxidase in white cabbage and apple trees.
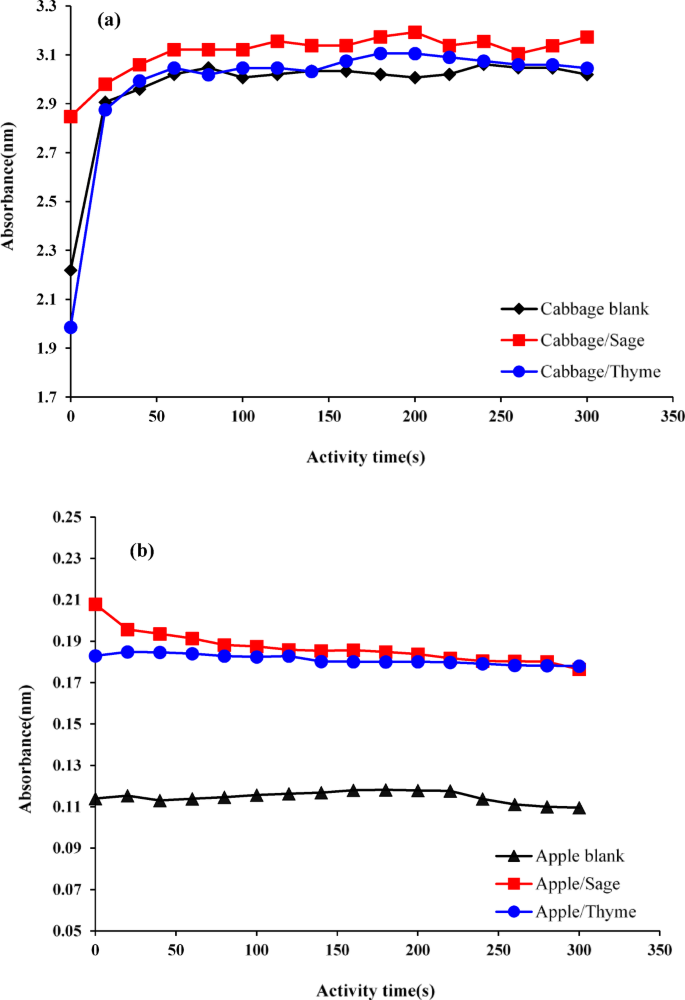
(a) Enzyme activity curve of white cabbage peroxidase under the influence of Thyme and Sage essential oils; (b) Enzyme activity curve of apple peroxidase under the influence of Thyme and Sage essential oils.
Sage EO significantly enhanced the activity of peroxidase in white cabbage, as indicated by the steeper slope of the absorption curve compared to the control. This suggests that Sage oil may act as an activator of peroxidase, improving the enzyme’s catalytic efficiency. The increase in enzyme activity could be attributed to specific interactions between the components of Sage oil and the enzyme, possibly leading to conformational changes that enhance its performance. This enhancement of peroxidase activity could be beneficial in agriculture, where increased enzyme activity might bolster plant defenses against environmental stressors and pathogens. Conversely, in food processing, where peroxidase activity influences oxidation reactions, the increase in enzyme activity might require careful management to avoid potential negative effects on product quality, such as discoloration or nutrient degradation.
Thyme EO exhibited a biphasic effect on peroxidase activity. Initially, the oil appeared to slow enzyme activity, as shown by the lower slope of the absorption curve compared to the control. However, over time, the enzyme activity increased, eventually surpassing that of the control. This complex response suggests an initial inhibitory interaction, likely due to Thyme oil components binding to the enzyme’s active site, temporarily reducing catalytic efficiency. As the interaction continues, either conformational changes or the removal of inhibitory components may lead to enhanced enzyme activity. Understanding this biphasic response is critical for applications in which precise control over enzymatic activity is essential, such as food preservation, where prolonged enzymatic activity may need to be regulated.
In apple trees, both Sage and Thyme EOs significantly increased peroxidase activity, indicating their potential as natural activators of plant defense mechanisms. This enhancement in peroxidase activity suggests that these EOs could be used in agriculture as natural elicitors to stimulate a plant’s defense response, potentially reducing the need for synthetic pesticides. However, the observed effects on enzyme activity also raise questions about the broader physiological impacts of EOs on plant growth, development, and fruit quality, all of which need to be further explored.
The study of peroxidase inactivation by encapsulated EOs provides valuable insights into their potential use in agriculture and food processing. The ability of EOs to modulate enzyme activity—either enhancing or inhibiting it—has far-reaching implications for these industries. These findings suggest that careful consideration of the specific EO and its interaction with enzymes is essential for optimizing its use in different applications.
MIC and MBC test results
The antimicrobial efficacy of encapsulated Thyme and Sage EOs was evaluated using MIC and MBC tests against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). While EOs are known for their antimicrobial properties, encapsulation enhances their stability and allows for controlled release, thereby improving their effectiveness. The MIC and MBC values obtained for both EOs are presented in Table 5, highlighting their potential as natural antimicrobial agents in various applications.
Thyme EO demonstrated potent antimicrobial activity, with MIC values of 4 µL/mL against E. coli and 2 µL/mL against S. aureus, and MBC values of 4 µL/mL for both bacteria. The high antimicrobial efficacy of Thyme oil is primarily attributed to its phenolic compounds, particularly thymol and carvacrol, which are known to disrupt bacterial cell membranes and interfere with essential cellular processes. Encapsulation of Thyme EO in nanocapsules further improved its antimicrobial performance by protecting it from degradation and enabling controlled release, maintaining sustained antibacterial activity over time. The low MIC and MBC values suggest that encapsulated Thyme EO could serve as an effective alternative to synthetic antibiotics, especially in applications where natural and sustainable antimicrobial agents are desired.
Sage EO exhibited antimicrobial activity, although it required higher concentrations than Thyme oil. The MIC values were 8 µL/mL for E. coli and 4 µL/mL for S. aureus, with MBC values of 16 µL/mL and 8 µL/mL, respectively. The lower antimicrobial efficacy of Sage EO compared to Thyme oil is likely due to differences in their chemical compositions. Sage oil contains a mix of terpenes, which, while possessing antimicrobial properties, are generally less potent than the phenolic compounds found in Thyme oil. Nevertheless, the encapsulated Sage oil still demonstrated significant antibacterial activity, making it a viable option for applications requiring a broader spectrum of activity.
Encapsulation of Thyme EO in HNSs showed superior antimicrobial performance compared to HPNs. The porous structure of the silica matrix allows for a slow, sustained release of the EO, maintaining its antimicrobial activity over an extended period. This sustained release is particularly advantageous in applications requiring long-term antibacterial effects, such as food preservation, medical coatings, and surface disinfection. The superior antimicrobial activity of Thyme oil encapsulated in silica emphasizes the importance of selecting appropriate encapsulation materials to enhance the functional properties of EOs.
Although HPNs also provided controlled release, their performance in enhancing the antimicrobial efficacy of encapsulated EOs was less pronounced than that of HNSs. This difference can be attributed to the structural properties of the polymers used, which may affect the release rate and stability of the EO. However, HPNs still offer potential advantages, such as biocompatibility, which could be beneficial in specific applications, including medical or pharmaceutical uses.
The MIC and MBC test results highlight the strong antimicrobial potential of encapsulated Thyme EO, particularly when incorporated into HNSs. These findings suggest that encapsulated EOs could serve as highly effective antimicrobial agents for a variety of industries, ranging from food preservation to healthcare, where natural and sustainable solutions are increasingly in demand.

:max_bytes(150000):strip_icc()/plants-that-repel-mosquitoes-4583885-hero-c3fbed5e21fd487ca4d9965ea0301980.jpg?w=768&resize=768,0&ssl=1)
:max_bytes(150000):strip_icc()/spruce-thyme-RenaLolivier-3241bfeed8264e19a60f3e80c64db4e2.jpg?w=768&resize=768,0&ssl=1)

:max_bytes(150000):strip_icc()/GettyImages-1469902775-f879f55a12ad4fc19db3e90790ac236d.jpg?w=768&resize=768,0&ssl=1)