
The air-dried powdered leaves of Thymus vulgaris (150 g) were pulverized and extracted with hexane at 45 °C for 6 h by a Soxhlet apparatus to yield a dark green extract (28.7 g, 19.2%). After hexane extraction, 121.3 g of defatted powder was weighed and extracted with chloroform at 45 °C for 6 h using the same apparatus with petroleum ether to yield a green extract (35.1 g, 28.9%). Finally, after chloroform extraction, 86.2 g of defatted powder was extracted with methanol at 45 °C with a Soxhlet apparatus for 6 h to yield a blue black extract (33.8 g, 39.2%). Based on their polarity, three organic solvents (hexane, chloroform, and methanol) were chosen; the average values of crude extracts percentage yield are calculated for each organic solvent.
Phytochemical screening results
Phytochemical screening tests conducted against all crude extracts indicated that hexane extracts constitute saponins, terpenoids, steroids, and cardiac glycosides. Chloroform extracts include saponins, terpenoids, phenols, and cardiac glycosides. Methanol extracts contain alkaloids, saponins, tannins, terpenoids, phenols, and cardiac glucosides. On the other hand, aqueous extracts contain saponins, alkaloids, terpenoids, phenols, and cardiac glycosides. The phytochemical screening test results for the hexane, CHCl3 and MeOH crude extracts of T. vulgaris showed the following results (Table 1).
Characterization of Tv-2: Compound Tv-2 was isolated from the CHCl3 crude extract of Thymus vulgaris and characterized. The Tv-2 compound was isolated as a reddish pink solid with an Rf value of 0.23 in hexane: ethyl acetate (3:1). Structure elucidation of the compound was based on the spectroscopic data obtained from FT-IR, NMR (1H-NMR and 13C-NMR) and UV–Vis spectral data. TLC (n-hexane: ethyl acetate, 3:1 v/v): Rf (0.23; 1H NMR (600 MHz, CDCl3: \(\delta\) 7.01 (d, J = 7.7 Hz, 1H) 6.70 (d, J = 8.9 Hz, 1H) 6.60 (s, 1H) 5.65 (s, 1H) 2.76 (m, J = 13.8, 6.8 Hz, 1H) 2.18 (s, 3H) 1.17 (d, J = 6.94 Hz, 6H); 13C NMR (600 MHz, CDC3): \(\delta\) 153.50, 148.33, 130.90, 121.31, 118.81, 113.20, 33.65, 23.95, 15.44; IR (KBr pellet): 3392 cm−1, 3018 cm−1, 2958 cm−1, 2885 cm−1, 1621 cm−1, 1582 cm−1, 1511 cm−1, 1503 cm−1, 1454 cm−1, 1421 cm−1, 1112 cm−1, 993 cm−1, 864 cm−1, 811 cm−1, 755 cm−1 : UV–Vis: \(\lambda\) max 274 nm. Experimental data obtained from FT-IR and UV–Vis were converted into spectral graphs using OriginPro 9.0 64-Bit software, while NMR (1H-NMR and 13C-NMR) spectral data were plotted using MestReNova.
FT-IR spectrum of Tv-2
The IR spectrum of Tv-2 is shown in Fig. 2. In the Tv-2 spectrum, clear characteristic absorption bands were observed at 3392 cm−1, which are characteristic of γOH groups associated with aromatic rings. The band at 3018 cm−1 was characteristic of the γC–H relationship characteristic of an aromatic ring. The band at 2958 cm−1 was characteristic of the γCH3 group, which was confirmed by an additional band at 2885 cm−1. The absorption band at 1621 cm−1 was characteristic of an absorption band of a conjugate double γC=C– bond. The intense absorption band at 1582 cm−1 was characteristic of a carbon skeleton with an aromatic structure and was more intense at approximately 1500 cm−1 (in this case, 1503 cm−1). The bands at 1582, 1511, 1503, 1454, and 1421 cm−1 were characteristic of aromatic rings, and the band at 1421 cm−1 was characteristic of γC–OH bonds in phenols. The bands at 1112, 993, and 864 cm−1 were characteristic of aromatic rings with substituents at positions 1, 2, and 5, respectively. The band at 811 cm−1 was typical for an m-substituted aromatic ring, and that at 755 cm−1 was typical for an o-substituted ring. From the conclusions drawn, it could be concluded that this compound belongs to the phenol group, which has a CH3 group in the o-position and a C(CH3)2 group in the m-position, which was confirmed by the structural formula of Tv-2.
The 1H-NMR (600 MHz, CDCl3) spectrum (Fig. 3) of compound Tv-2 showed the presence of aromatic, alkene, alcohol hydroxyl, and aliphatic protons. The aromatic part of this compound contains three protons that are chemically nonequivalent. These three signals appeared at δ 7.01 (1H, d (J = 7.7 Hz)), δ 6.70 (1H, d (J = 8.9 Hz)) and δ 6.61 (1H, s). The signal at δ 5.65 indicated the presence of a hydroxyl proton, which is directly attached to the benzene ring. Moreover, the spectrum of this compound shows δ 2.76 (1H, m (J = 13.8, 6.8 Hz)) and δ 2.18 (3H, s), which indicates the presence of two different methane and methyl protons, both of which are attached to the phenyl ring. In addition, the spectrum of this compound shows two 3H doublets at δ 1.17 (J = 6.94 Hz), which revealed the presence of two methyl protons.
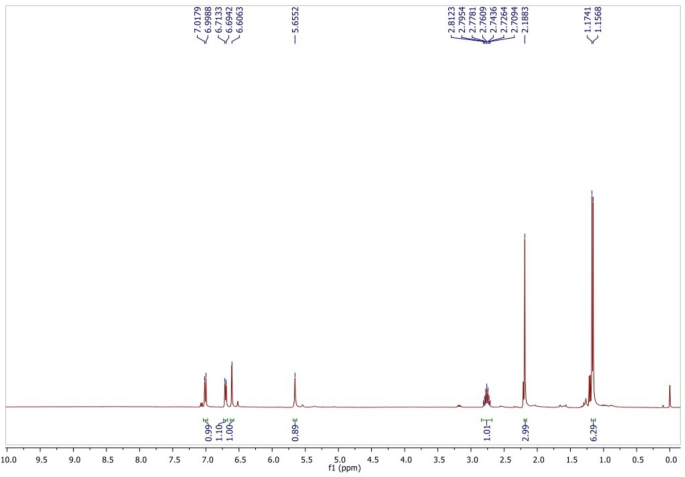
1H-NMR spectral data of Tv-2 in CDCl3.
Figure 4 shows the 13C NMR spectrum of compound Tv-2, which shows well-resolved aromatic carbon, hydroxyl carbon and nonoxygenated aliphatic carbons. 13C-NMR of the compound revealed that the Tv-2 compound has ten carbon atoms. In the aromatic region, there are six nonequivalent carbon signals at δ 113.20, 118.81, 121.31, 130.90, 148.33, and 153.50. In the aliphatic region, there is one carbon signal at δ 33.65, which is a methine carbon directly attached to the phenyl ring. The peak at δ 23.95 represented two aliphatic carbons that are chemically equivalent. Additionally, the signal at δ 15.44 indicated the presence of a methyl carbon attached to the aromatic ring. Therefore, the 13C NMR data showed the presence of aliphatic, alcoholic and aromatic carbons that exactly matched the previously identified FT-IR functional groups.
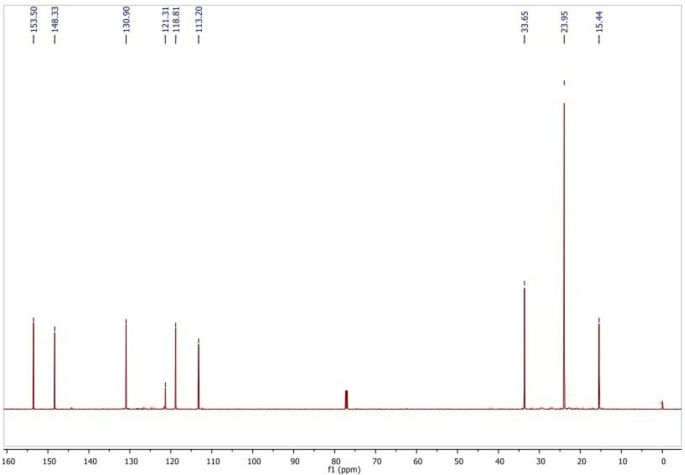
13C-NMR spectral data of Tv-2 in CDCl3.
The UV–Vis spectrum of Tv-2 (Fig. 5) shows an absorption band at 274 nm, indicating the presence of an aromatic ring attached by a hydroxyl functional group through a π–π* electronic transition.
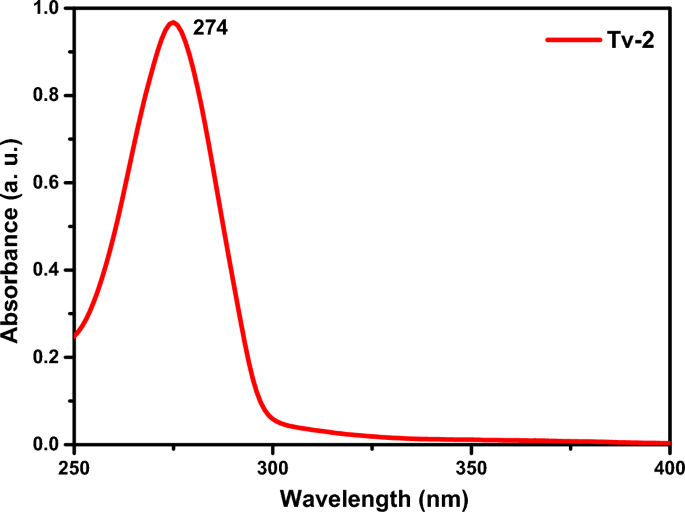
UV–Vis spectral data of Tv-2.
Based on the above UV–Vis, FT-IR, and NMR spectroscopic data, the structure of Tv-2 was determined to be 5-isopropyl-2-methylphenol (Fig. 6).
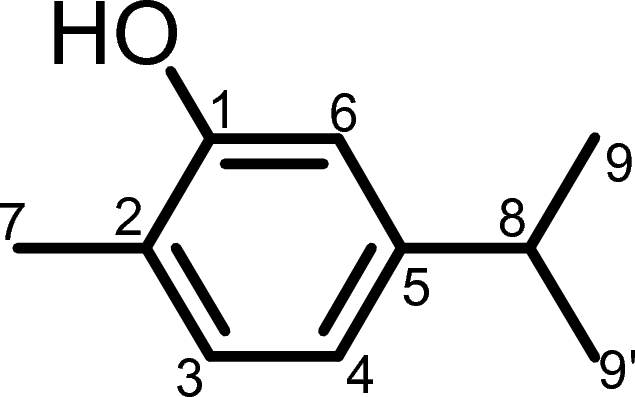
The proposed structure of Tv-2.
After obtaining the 13C NMR spectral data, we utilized the CSEARCH spectral similarity search platform to determine the structure of Tv-2. Among the one hundred thirty-six organic compounds generated from CSEARCH, carvacrol has been proposed to be one of the most promising compounds31. Previous research has extensively explored the chemical composition of this medicinal plant. Studies have consistently identified carvacrol as a major constituent of the essential oils extracted from various cultivars of Thymus vulgaris32. Furthermore, chemical analysis of Thymus vulgaris essential oil has consistently revealed the presence of carvacrol in combination with thymol33. Additionally, isolation techniques involving hydrodistillation, fractionation, and purification have been employed to extract carvacrol from Thymus vulgaris, along with other compounds such as thymol and linalool34. Carvacrol, also known as 5-isopropyl-2-methylphenol, is one of the components identified in T. vulgaris and is commonly referred to as Tv-2.
Antimicrobial assay
The hexane crude extract, chloroform crude extract, methanol crude extract and some isolated fractions from Thymus vulgaris showed the following results of inhibition against five bacterial strains one gram-positive bacterium, Staphylococcus aureus ATCC 29,213 and four gram-negative bacteria Escherichia coli ATCC 25,922, Acinetobacter baumannii BAA 1605, Pseudomonas aeruginosa ATCC 27,853, and Klebsiella pneumoniae BAA 1705 using the agar dilution method (Table 2).
Extracts of Thymus vulgaris showed significant antibacterial activity against pathogenic bacteria. The MICs of T. vulgaris hexane crude extract, chloroform crude extract, methanol crude extract, fraction-1, and fraction-2, which are Tv-2, fraction-3, fraction-4, fraction-5, and fraction-6, for all the bacterial strains were greater than 64 μg/mL. Sharif and Shallal separately conducted research demonstrating that Thymus vulgaris oil exhibits potent antimicrobial activity against Streptococcus sanguinis, Porphyromonas gingivalis, and Prevotella intermedia35,36, Cutibacterium acnes, Staphylococcus epidermidis37. Researches showed that Thymus vulgaris bioactive compounds are effective against various microbes such as Escherichia coli, Salmonella typhimurium, Enterococcus faecalis, Staphylococcus aureus, and methicillin-resistant Staphylococcus aureus38, Candida albicans, and candida famata, commonly found in urinary tract infections in dogs and cats39, mammaliicoccus lentus and salmonella thyphi40. Based on Galgano et al., in vitro test Thymus vulgaris essential oil exhibited complete inhibition growth of Escherichia coli and Staphylococcus aureus41. According to Antih et al. and his coworkers, Thyme essential oil also shows antibacterial activity against respiratory pathogens such as Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pyogenes in both the liquid and vapor phases42. However, our results were not in accordance with those of other reports. This discrepancy may be due to differences in the samples used, the extraction process, the composition of the samples used and the solubility process (water or solvents). Researchers suggest that the antimicrobial activity of bioactive components from Thymus vulgaris relies on both the quantity of bioactive components and the specific types of microorganisms involved43.
Conclusion and future perspectives
The extraction of natural products using Soxhlet apparatuses has attracted increasing interest because of its categorization as a conventional method during the past decade, with increasing attention given to environmental, economic and safety considerations. The three organic solvents hexane, chloroform, and methanol were used to successively extract the thyme herb using a Soxhlet apparatus based on their polarity differences. The chloroform crude extract was subjected to column chromatography using increasing polarities of hexane and ethyl acetate mixtures, which resulted in the isolation of thirteen fractions, including the characterized compound 5-isopropyl-2-methylphenol, from fraction 2 by using different spectroscopic techniques. These findings indicate the potential of Thymus vulgaris crude extract as a natural antibacterial agent with broad-spectrum activity against various pathogenic bacteria. We hope that other researchers will be inspired to collect all the information required in this field by what is missing in our research paper to explore additional applications by synthesizing nanoparticles for conducting biosensors and evaluating the cytotoxicity and antioxidant activities of T. vulgaris.

:max_bytes(150000):strip_icc()/plants-that-repel-mosquitoes-4583885-hero-c3fbed5e21fd487ca4d9965ea0301980.jpg?w=768&resize=768,0&ssl=1)
:max_bytes(150000):strip_icc()/spruce-thyme-RenaLolivier-3241bfeed8264e19a60f3e80c64db4e2.jpg?w=768&resize=768,0&ssl=1)

:max_bytes(150000):strip_icc()/GettyImages-1469902775-f879f55a12ad4fc19db3e90790ac236d.jpg?w=768&resize=768,0&ssl=1)